What Is the Density of Krypton Gas at Stp
Krypton from Ancient Greek. 7 rows Density of Krypton.

Aim How To Convert From Moles To Volume And Vice Versa Ppt Download
Calculate the density of Xenon gas Xe at STP.

. What is the percent composition of this compound. The density of krypton at STP is 374 kgm3 or 000374 gcm3 at STP of 0oC 27315 K and 1 atm. Problem 23 page 322 374 gL.
At STP thepressure of natural gas. What is the density of Krypton Kr gas in gL at STP. Krypton weighs 00037493 gram per cubic centimeter or 37493 kilogram per cubic meter ie.
Density of natural gas at STP. The density of krypton in its liquid state is 2155 gmL. Krypton like the other noble gases is.
Information you will need. Which label belongs to the area marked z. At 0C 32F or 27315K at standard atmospheric pressure.
Atomic Mass amu 83798. Typical densities of various. The calculated density is 00037386 gcm3.
The density of Kr at STP is value1 gL. A compound is formed when 903 g Mg combines completely with 348 g N. Electron Configuration Ar 3d10 4s2 4p6.
How to solve the problem. Why is krypton a noble gas. 000112 torr 6688 torr B94 torr 646 torr vk Which of the following is considered the.
Assume ideal conditionsPVnRTnmM nmoles. Problem 33 page 326 722 Mg 278 N. Density at STP gcm3 375.
When liquid at bp. Density of Krypton is 375gcm 3. Chen drew a diagram to compare the ways in which different organisms obtain nitrogen.
In Imperial or US customary measurement system the density is equal to 023406 pound per cubic foot lbft³ or 000216723 ounce per cubic inch ozinch³. 1st Ionization Energy eV 139996. STP stands for Standard Temperature and Pressure.
1 Show answers Another question on Chemistry. Density is mass over volume. Electron Affinity kJmol Electronegativity Pauling scale 3.
Kryptos the hidden one is a chemical element with the symbol Kr and atomic number 36. The density of krypton at STP is 374 kgm3 or 000374 gcm3 at STP of 0oC 27315 K and 1 atm. 3 rows Density at STP 3749 gL.
Calculate the density of CH4 gas at STP. Density of CO2 at STP 4401 gmol divided by the 224liters196 gramsLiter. The density in its solid phase is 309 gcm3 and in its gas phase is 3425 gL.
The atom fluorine generally will become. Density of krypton is equal to 37493 kgm³. O 00250 gL O 374 gL 178 L 0267 gL If the atmospheric pressure in Denver is 085 atm then what is this in torr 1 atm - 101325 Pa- 760 torr 1 torr 1 mmHg.
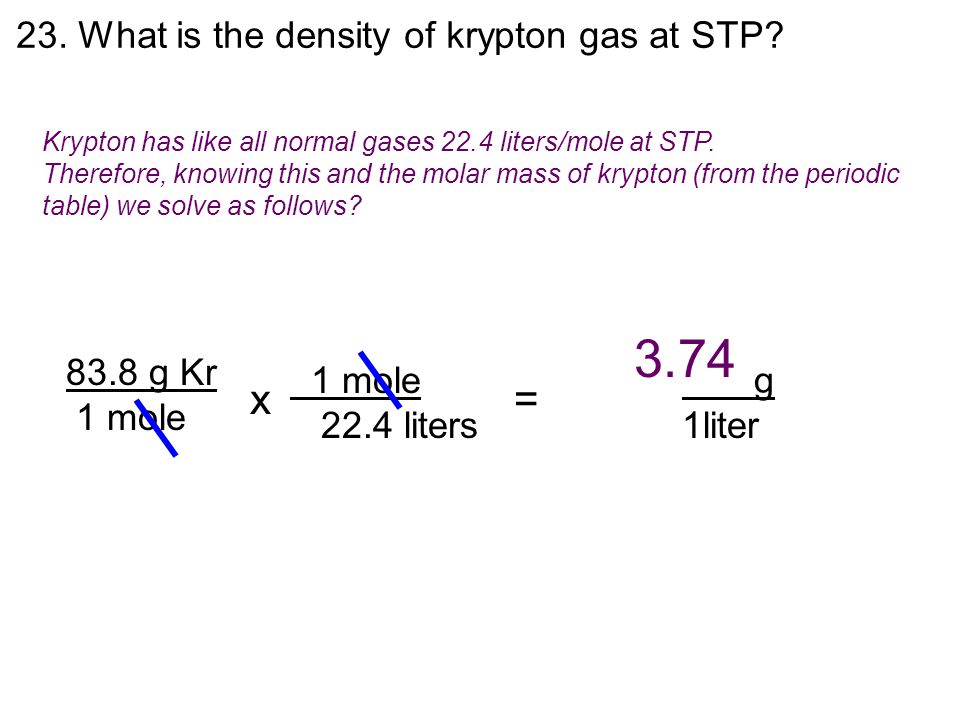
D MV this is what we need to find. What bonds keep krypton in solid phase. What is the density of krypton gas at STP.
What is the density of krypton gas at stp. Assuming ideal behavior what is the density of krypton gas at STP in gLIR -008206 LatmK mol. It is a colorless odorless tasteless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lampsWith rare exceptions krypton is chemically inert.
2413 gcm 3. Molecular weight of CH4 1605 gmol STP Pressure P 1 atm unit of pressure and Temperature T à 273 K unit of temperature Use the ideal gas law. The calculated density is 00037386 gcm3.
What is the density of krypton gas at STP.

Chemical Quantities Stoichiometry Ppt Download

Chapters 10 Chemical Quantities Ppt Download
Molecules Grams Moles Finish Here Start Here 1 27 Correct Units Ppt Video Online Download
Comments
Post a Comment